Hydrolysis of Polyesters
Polyesters can undergo hydrolytic main chain scission to form water and soluble fragments. The loss of molecular weight has a dramatic effect on the service life and mechanical properties even if only one to two percent of the ester units are hydrolized.1 At room temperature, this reaction is rather slow, whereas at temperatures above the softening or melting point the rate of hydrolysis increases rapidly. The effect is greatly accelerated when a catalyst like hydrochloride acid (HCl) or p-toluenesulfonic acid (p-TSA) is present. As has been shown by Szabo-Rethy et al. (1972), the critical water concentration for rapid acid catalyzed hydrolysis of poly(ethylene terephthalate) (PETE) is in the range of 2 percent for p-TSA.2 Thus, exposure to humidity has to be avoided during melt processing of polyesters and its copolymers whereas exposure to humidity during service life should be restricted to neutral conditions. The process of (acid catalyzed) hydrolysis of ester bonds can also be utilized to recover monomers or to tailor polymers that have to be biodegradable in moist environments (compostable plastics)
The most important commerical polyester is poly(ethylene terephthalate) (PET, PETE). It finds uses as fibers, films, and injection molded parts. Particularly PET bottles are of interest in regard to recycling due to the large production volume.3
The hydrolysis of polyesters is rather slow under neutral conditions (pH ≈ 7), but is greatly accelerated when an acid or a base is present because both act as a catalyst as can be seen below.
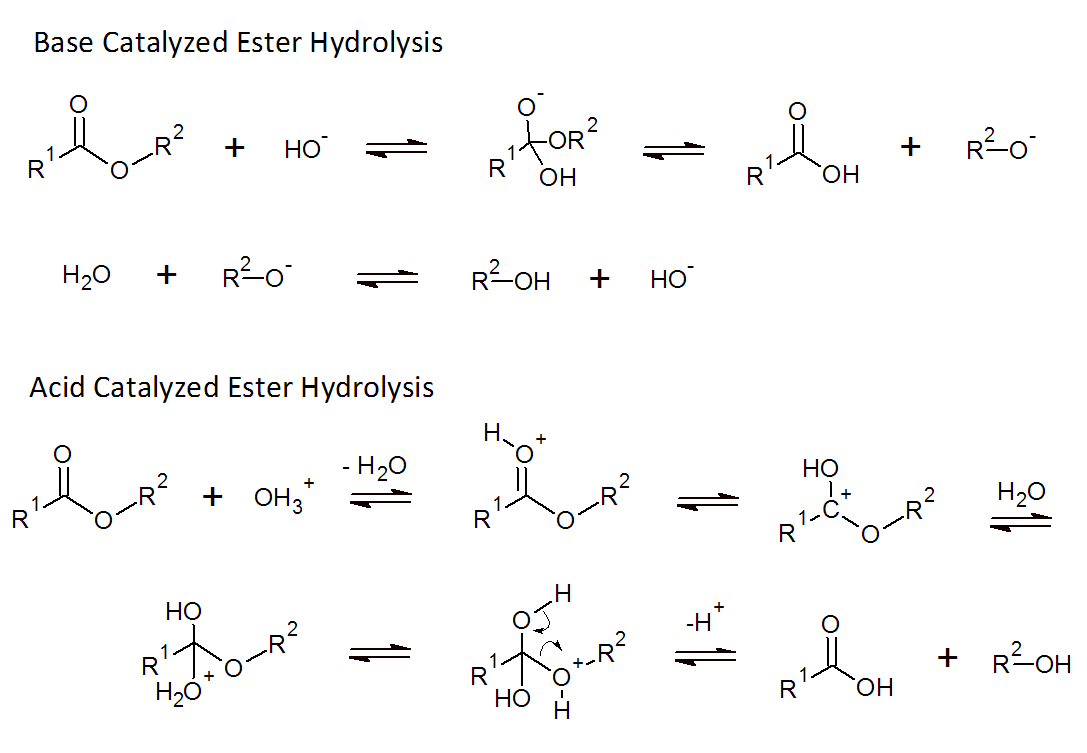
In some cases, the chain ends are acid groups which also act as catalysts. The hydrolysis will produce additional acid groups. Thus, the reaction rate will steadily increase over time. This process is called autocatalysis or auto-accelerated hydrolysis. The reaction rate of this process is proportional to the concentration of ester groups and water as well as the concentration of acid:
where [H2O] is the water concentration and [H3O+]
the hydrogen ion (hydronium) concentration which depends on the overall number of acid
groups. [COOR] is defined as
the mole equivalent of ester groups per unit volume.
This simplification is only valid if we assume that all polymer
chains have the same rate constants ka
regardless of size (molecular weight) of the polymer chains.
If
a base is present, we
also have to include a base-catalyzed hydrolysis
reaction:
In this equation, ka and kb are the second order rate constants of acid-, and base-catalyzed hydrolysis.4 Since both the catalyst and the water content generally remain constant, the equation above can be simplified to
The product of [H3O+] and [-OH] is constant at equlibrium. Thus
and
where Kw is the ionization or dissociation constant of water. This requations shows that the overall rate of hydrolysis strongly depends on the pH, meaning a slight change in pH causes a singnificant change in the rate of reaction.
All ester reactions are equilibrium reactions, meaning the reaction will not result in completely hydrolyzed polymer unless the reaction products are continuously removed. The concentrations at equilibrium will depend on the rate constant for esterification (ke) and depolymerization or hydrolysis (kh). The ratio of these two constants is the equilibrium constant K:
where [COO]E, [COOH]E and [OH]E are the mole equivalents of ester, acid, and alcohol groups per unit volume and p is the extend of reaction. Since the average degree of polymerization equals Xn = 1 / (1 - p), the equation above can be rewritten
K = p Xn2 [H2O] / M0 = (Xn - 1) Xn [H2O] / M0
or
Xn ≈ (K M0 / [H2O])½
Thus, the equilibrium molecular weight is inversely proportional to the square root of the water content in the polymer [H2O].
Notes and References
-
J.E. Pickett, D.J. Coyle, Polymer Degradation and Stability, Vol. 98, Isssue 7, Pages 1311-1320 (2013)
Szabo-Rethy and Vanco-Szmercsanyi, Chem. zvesti. 26, Pages 390-396 (1972)
-
Mechanical recycling can lead to some degradation due to thermal and mechanical stress which could result in lower mechanical properties and lower transparency.
-
Often a third term for uncatalyzed hydrolysis is included. However, this mechanism is only important at very low hydrogen ion concentration (pH ≈ 7) because its rate constant is much smaller than those of acid- or base-catalyzed hydrolysis.
-
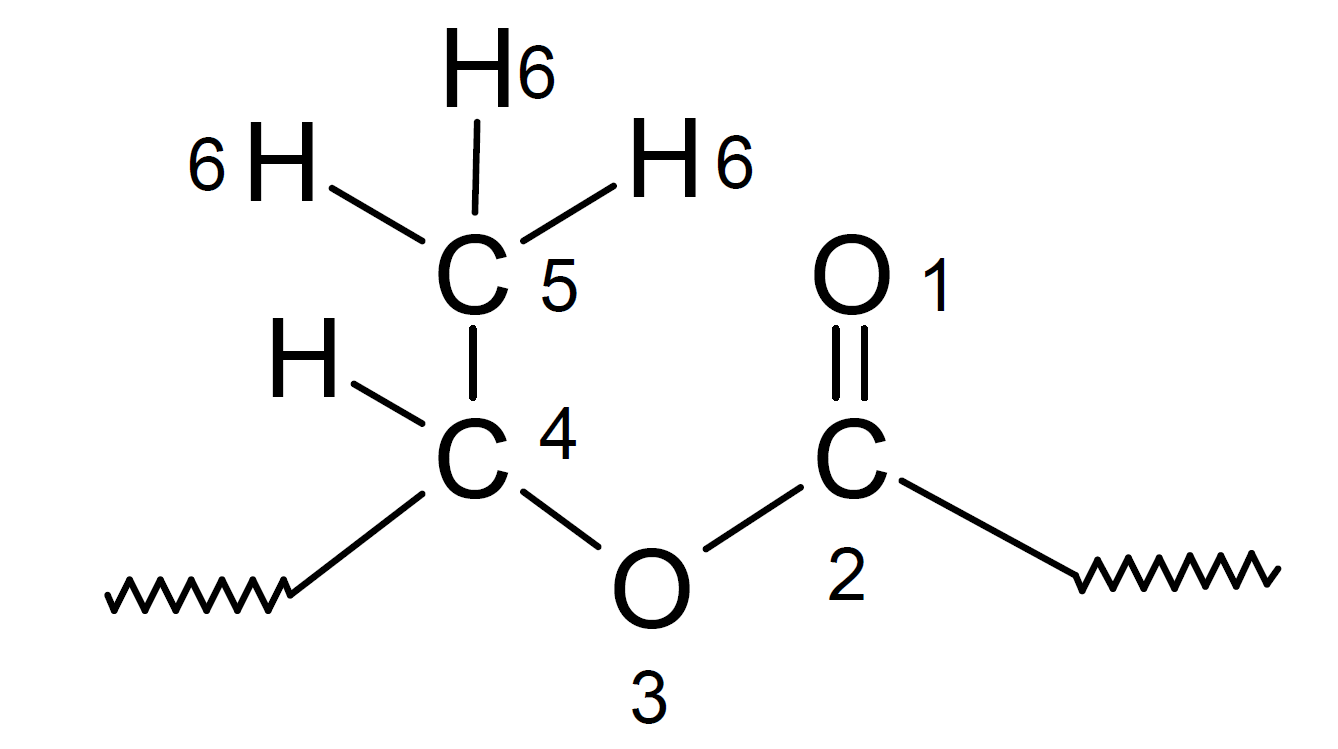
The rate of hydrolysis also depends on the stereochemical composition of the chain. The hydrogen, oxygen and carbon atoms of a group will interfere sterically with rotations of other groups which will limit the number of conforamtions which allow attack by hydroxyl and hydronium ions. Particularly stable are polyesters with a large number of atoms in the sixth position. This empirical rule is known as Newman's rule of six.6
A. Lopalco, J. Douglas, N. Denora, V.J. Stella, J. Pharm. Sci. 105, 2, 664-672 (2016)